《中国糖尿病杂志》官方网站

来自:中国糖尿病资讯网 编辑:admin|点击数:|2011-12-01
基金项目:国家高技术研究发展计划资助项目(2011AA02A116)
作者单位:100730北京,卫生部北京医院卫生部临床检验中心
通讯作者:陈文祥,E-mail:wxchen@nccl.org.cn
张传宝 闫颖 赵海舰 张江涛 马嵘 张天娇 王冬环 曾洁 陈文祥
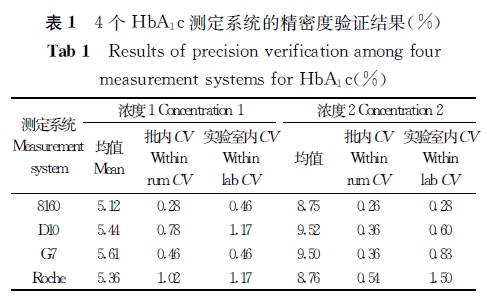
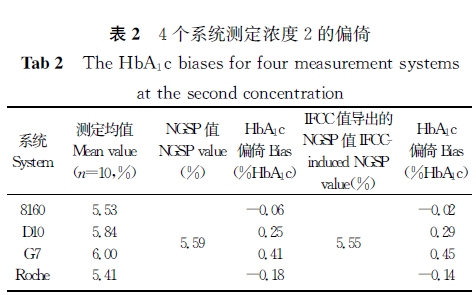
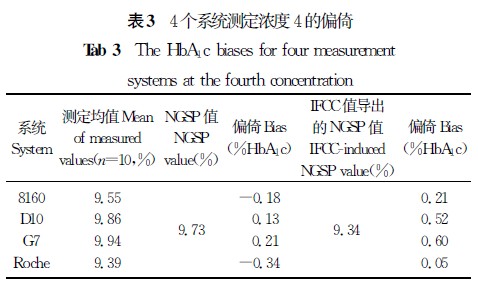
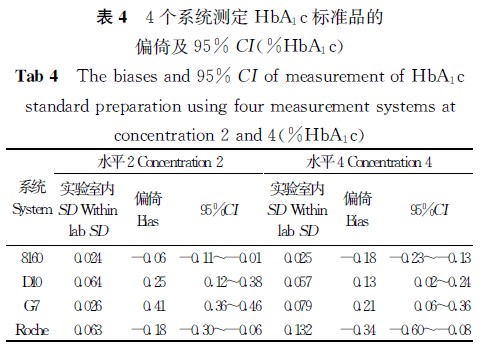
【摘要】 目的 验证4个HbA1c测定系统的正确度、精密度,以判断是否符合相关要求,为临床实验室的使用提供相关依据。 方法 采用一种简便的实验方案,在5 d内,对3个基于离子交换高效液相色谱法和1个基于免疫比浊法原理的HbA1c测定系统进行精密度和正确度验证实验,计算各个系统的批内精密度、实验室内精密度及测定结果的正确度。 结果 4个系统的批内精密度CV为0.26%~1.02%,实验室内精密度CV为0.28%~1.50%;4个系统测定标准物质的均值[美国国家HbA1c标准化计划(NGSP)值]和标准物质定值的偏倚为-0.34% HbA1c~0.41%HbA1c,与国际临床化学与检验医学联合会(IFCC)定值导出的NGSP值的偏倚为-0.14% HbA1c~0.60%HbA1c。 结论 4个系统的批内精密度和实验室内精密度及正确度性能符合相关要求,能满足临床工作的需要。
【关键词】糖尿病;糖化血红蛋白;免疫比浊法;离子交换高效液相色谱法
doi:10.3969/j.issn.1006-6187.2011.11.002
注:主要分析系统和设备
1.日本东曹(TOSOH)HLC-723 G7 HbA1c测定仪及其配套试剂及校准品(简称G7),离子交换高效液相色谱法。
2.日本爱科来(Arkray)ADAMSTMA1c HA-8160 HbA1c测定仪及其配套试剂及校准品(简称8160),离子交换高效液相色谱法。
3.美国伯乐(Bio-Rad)D-10 HbA1c测定仪及其配套试剂和校准品(简称D10),离子交换高效液相色谱法。
4.日立7170A自动生化分析仪/罗氏(Roche)免疫比浊法HbA1c试剂盒及校准品(简称Roche)。
Verification of performance for precision and trueness of four HbA1c measurement systems
ZHANG Chuan-bao, YAN Ying, ZHAO Hai-jian, et al. Beijing Hospital, National Center for Clinical Laboratories, Beijing 100730, China
Corresponding author: CHEN Wen-xiang,E-mail: wxchen@nccl.org.cn
【Abstract】 Objective To verify the performance for precision and trueness of 4 glycated hemoglobin measurement systems, to determine whether relevant requirements are fulfilled, and to provide guidelines for clinical laboratory staff and clinicians. Methods A simple experiment protocol was applied to verify in five days the performance for precision and trueness of 4 HbA1c measurement systems, three of which were based on ion-exchange high-performance liquid chromatography and one based on immune turbidimetric assay, Within-run and within-lab precisions and trueness were calculated and evaluated against requirements. Results The within-run precision and within-laboratory CVs of four systems were 0.26%~1.02% and 0.28%~1.5% respectively. The bias of four systems were -0.34% HbA1c ~ 0.41% HbA1c (NGSP value) and -0.14% HbA1c ~ 0.61% HbA1c(value derived from the IFCC- NGSP equation). Conclusions The performance of within-run and intra-lab precision and trueness of 4 systems meets relevant requirements and clinical needs.
【Key words】 Diabetes;Glycated hemoglobin;Immune turbidimetric assay;Ion-exchange high-performance liquid chromatography
版权所有:《中国糖尿病杂志》社 主管单位:中华人民共和国教育部 主办单位:北京大学
地址:北京市西城区大红罗厂街1号 邮编:100034 电话(传真):010-88505683
中国糖尿病杂志社版权所 京ICP备11029051号-1 Powered by JiuduCMS 技术支持:九度创想